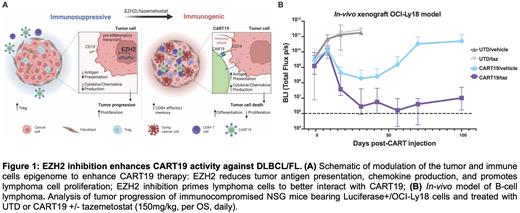
Introduction: Anti-CD19 chimeric antigen receptor T cell (CART19) immunotherapy provides a high response rate in diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL) patients, but only ~30-40% of patients maintain a durable remission. The factors contributing to lymphoma resistance to CART19 are numerous, multifaceted, and coincidental, such as tumor intrinsic properties, CART dysfunction, the immunosuppressive tumor microenvironment (TME), and host factors. However, most strategies currently in development to increase CART efficacy only address one resistance mechanism at a time; therefore, it is unlikely that these approaches will be consistently effective in a group of heterogeneous patients. In this study, we sought to develop a strategy to overcome multiple resistance mechanisms simultaneously. Epigenetic modulation offers a promising approach to this goal, as it can reshape the tumor landscape, as well as immune cells. DLBCL/FL cells depend for their survival on the methyltransferase EZH2, which represses gene expression by generating histone 3 lysine 27 trimethylation. Interestingly, somatic gain-of-function mutations of EZH2 are frequently found in DLBCL/FL and drive lymphomagenesis and impair tumor B-cell to T-cell interactions, leading to an immune evasive phenotype. Therefore, we hypothesized that EZH2 inhibition could enhance CART anti-tumor effect, remodeling the entire tumor ecosystem by: a. inhibiting lymphoma growth; b. increasing lymphoma immunogenicity; and c. enhancing T cell function ( Fig. 1A).
Methods and Results: Through short-term killing assays, we compared CART19 alone to CART19 combined with the EZH2 inhibitor tazemetostat (taz), and demonstrated that taz enhances CART19 tumor killing of DLBCL cell lines [OCI-Ly18 and Toledo (with wild-type EZH2) and SU-DHL-4 (with mutant EZH2)]. Interestingly, the highest effect was observed when tumor cells were pre-incubated with taz and taz was kept in the coculture with CART19. This finding underscores the importance of tumor priming to improve CART19 recognition and activation. Since EZH2 is also expressed by T cells, we tested if EZH2 inhibition impacted CART cell function. Co-treatment of lymphoma cell lines with taz and CART19 was associated with higher cytokine secretion over time (INFy and TNF) by CART cells and CART cell expansion. Most importantly, using the sub-cutaneous OCI-Ly18 xenograft lymphoma model in immunodeficient NSG mice, we demonstrated that taz administration improved tumor control ( Fig. 1B) and CART19 expansion and infiltration in vivo.
Mechanistically, we studied the molecular changes in lymphoma cells (OCI-Ly18 and SU-DHL-4) induced by exposure to taz/CART19 or CART19 alone by performing RNA sequencing. We observed upregulation of genes involved in inflammatory response, B-cell activation/differentiation (e.g., BATCH2), adhesion (OX40L, CD80), and motility (CAECAM1, NRCAM1, EPHB2, CXCL16, CXCL10), sustaining the hypothesis that EZH2 inhibition changed lymphoma immunogenicity and the tumor:CART cell interaction. Indeed, functionally, we observed that taz exposure increased tumor:CART cell interaction in vitro as demonstrated by increased avidity (zMovi assay). Further, single-cell RNA sequencing performed on circulating CART19 cells from taz-treated-mice revealed enrichment in naïve and less exhausted CART cells.
Lastly, we assessed whether increased activation of EZH2 in tumor cells reduces responses to CART19 in the clinic. We retrospectively analyzed a cohort of large B-cell lymphoma patients treated with commercial CART19 (tisagenlecleucel and axicabtagene ciloleucel) at the University of Pennsylvania between January 2018 and March 2023 for response (Lugano criteria) according to their EZH2 mutational status. Patients harboring EZH2 activating mutations (14/69) had a reduced complete response rate to CART19 as compared to wild-type patients (15.4% vs. 40.0%).
Conclusions: Our data demonstrate that modulation of EZH2 enhances CART19 immunotherapy in preclinical models of lymphoma. EZH2 inhibition rewires lymphoma immunogenicity, making tumor cells more susceptible to CART19 killing and reducing CART19 terminal differentiation. By understanding and modulating the interplay between tumor and CART cells, we can unlock the full potential of CART19 immunotherapy in DLBCL/FL patients, aiming at long-lasting treatment outcomes.
Disclosures
Ghilardi:viTToria biotherapeutics: Consultancy. Patel:viTToria biotherapeutics: Consultancy. Schuster:TG Therapeutics: Research Funding; Adaptive Biotechnologies: Research Funding; Abbvie: Research Funding; Juno Therapeutics: Research Funding; DTRM: Research Funding; Merck: Research Funding; Pharmacyclics: Consultancy; Nanovecter: Consultancy; Celgene: Consultancy, Research Funding; BiGene: Consultancy; Acerta: Consultancy; Loxo: Consultancy; Legend Biotech: Consultancy; Janssen: Consultancy; Genentech/Roche: Consultancy, Research Funding; Incyte: Consultancy, Research Funding; MustangBio: Consultancy; Morphosys: Consultancy; Nordic: Consultancy; Regeneron: Consultancy; Novartis: Consultancy, Research Funding. Melnick:Janssen: Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Treeline Biosciences: Consultancy; Ipsen: Consultancy, Research Funding. Ruella:GlaxoSmithKline: Consultancy; Bayer: Consultancy; viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; Bristol Myers Squibb: Consultancy; NanoString: Consultancy, Research Funding; Beckman Coulter: Research Funding; AbClon: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal